*견적이나 기타 궁금하신 사항은 게시판을 이용해 주십시요.^^
제품명 : PHARMACEUTICAL Autoclaves SERIES
(250,450,1040,2000,LITERS) PHARMACEUTICAL CARE
제조사 : TUTTNAUER / ISRAEL
-FEARURES-
Pharmaceutical Autoclaves Models
cGMP Pharmaceutical autoclave with fully automatic sliding doors designed to
conform to end-user requirements (URS) using steam as the sterilizing agent,
appropriate for pharma production and quality assurance processes. The T-Max
12 autoclave chamber volume is 1040 liters.

| Model |
Chamber
Dimensions
( Wx H x D)
mm |
Chamber
Volume (Liter) |
Door
Options
5596 |
| T-Max 12 |
660 x 1220 x 1295 |
1040 |
Horizontal sliding - 1 or 2
doors |
Autoclave
Features and Options
Standard ✓ Optional
o
| Pressure
Vessel |
| AISI 316L stainless
steel chamber |
✓ |
|
ASME Code Section VIII, Division 1
or PED 97/23 EEC |
✓ |
| AISI 316L stainless steel jacket |
o |
| Polished Surface (Ra less than 0.4 μm) |
o |
| Insulation encased in stainless steel |
✓ |
|
Jacket covering 100% of the chamber wall for
uniform heat distribution. |
✓ |
| Jacket cooling |
o
|
| Bio-shield preperation |
o
|
| AISI 304 or 316L stainless steel base frame |
o
|
| Door(s) |
| Automatic sliding door(s) |
✓ |
| Bi-directional door sequencing |
o
|
| Piping
& Components |
|
Pharmaceutical grade sanitary piping and
components (primary) |
o |
Pharmaceutical grade sanitary piping and
components (primary & secondary) |
o |
| PT100 temperature sensor in drain |
✓ |
| Sanitary 0.2 μm air admission filter |
o |
| Chamber water level & alarm |
✓ |
|
Isolated pressure (membrane) gauges |
o |
| Videoboroscoping report for clean piping |
o
|
| Air detector |
o
|
| Other |
| Clean steam generator |
o |
| Biohazard system for waste treatment |
o |
| Filter integrity testing connections |
o
|
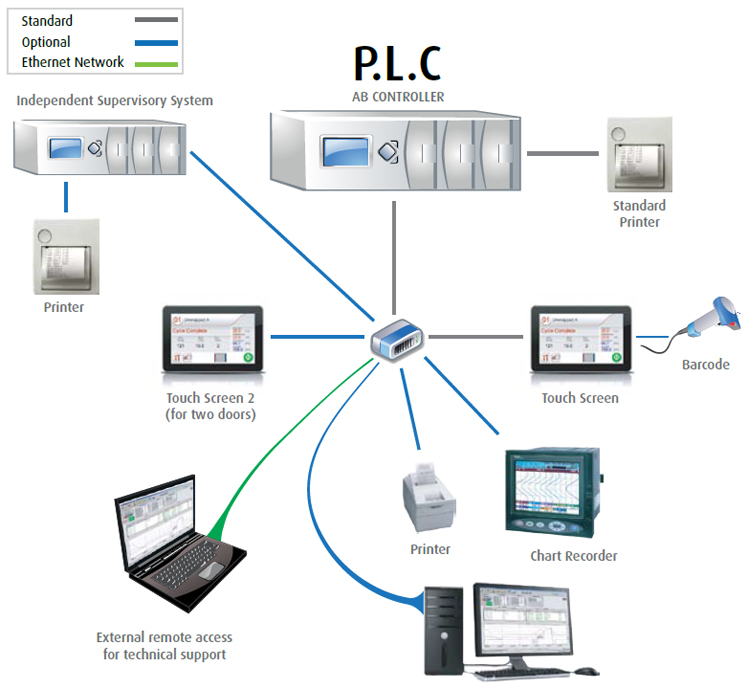
CONTROL SYSTEM
Advanced Control System
Take advantage of Tuttnauer’s sophisticated user-friendly PLC control system
based on the advanced Allen-Bradley platform in all pharmaceutical
autoclaves.
Standard Features
- 7" Multi-color touch screen for easy access to controls and information via
the panel
- Stores the last 200 cycles in built-in memory
- 4 access levels and 11 user passwords to control access/operation of the
autoclave
- In/Out test (enables technician to check each system component separately)
- Sterilization Temperature range 110°C to 137°C
- Ethernet connection for remote monitoring, remote maintenance, and software
updates
- Filter replacement notifications based on the number of cycles
- 21 CFR part 11
Optional Features
- 10" Multi-color touch screen
- F0 software control
- Up to 16 different Barcodes

Sophisticated Touch Screen HMI
The Human Machine Interface (HMI) has been designed with the following
considerations:
- Multi-color display for easy reading
- Easy operation
- Quick access to important information
- 26 Multiple languages
- Built-in view of historical cycle data
- Graphical display of Temperature and Pressure trend graphs
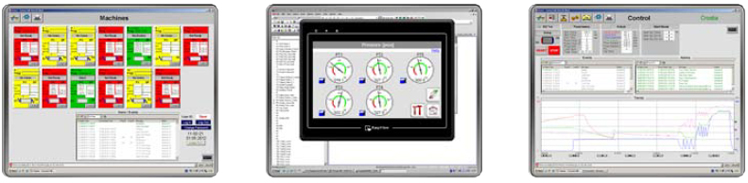
SCADA Software & Hardware
SCADA software (optional) allows for control and monitoring of up to 16
sterilizers on an external PC. The software retrieves data, creates graphs,
tables and printouts. Thousands of hours of cycle data can be stored in the
cycle history.
PROGRAMS
The advanced control system allows for customization of each program which
allows for a high degree of versatility, providing the flexibility of using the
same autoclave for many types of loads and applications. The controller has up
to 22 programs.
Tuttnauer thoroughly checks each program to ensure that they perform
according to our customers requirements (URS).
Unwrapped & Solid
- Utensils
- Glassware
- Machine Parts
Wrapped & Porous Goods
- Textiles
- Filters & Filter Vessels
- Elastomers & Rubbers
- Ampoules & Vials
- Instruments & parts (wrapped)
- Glassware (Wrapped)
Liquids & Media (Optional)
- Liquids (open)
- Liquids (sealed)
Biohazard (Optional)
- Pathogens
- Biohazard Waste
Test & Control
- Leak Test
- Bowie & Dick Test
- Filter Sterilization
- Filter Integrity Testing Connections (optional)

Design and Construction
The pharmaceutical autoclave line is designed and constructed for sanitary
pharmaceutical applications which require strict compliance with cGMP. All
machine components exposed to steam are made from 316L stainless steel.

Diaphragm Valves
Diaphragm valves and gauges are used to allow maximal drainability and
minimise the risk of contamination.

Specialized Piping
To meet the high sanitary requirements of the cGMP the pharmaceutical-grade
sanitary line (primary piping) to the chamber steam inlet is orbital
welded.

Sanitary Air Filter
A 0.2 μm air filter ensures that bacteria free air enters the chamber. A SIP
provision is available for the sanitary filter.

Air Detector
An optional air detector detects insufficient air removal and non-condensable
gases in the steam.
Chamber and Jacket Construction
The 316L Stainless Steal chamber inner walls have a mirror like surface level
polish less than 0.4 μm (specific polishing values are available upon request).
The chamber is constructed to be drainable with smooth, rounded and sloped
surfaces to enable proper drainage and cleaning.
The jacket is constructed from 316L stainless steel. The chamber is cooled
through jacket water cooling.




















